If they do identify the elements that undergochanges in oxidation number. If they do identify the elements that undergo changes in oxidation number.
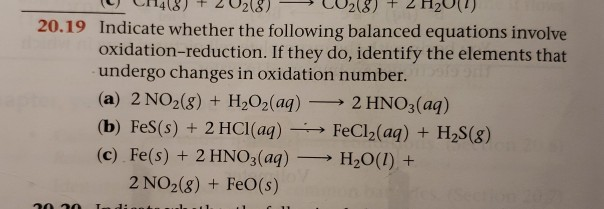
Solved 20 19 Indicate Whether The Following Balanced Chegg Com
If they do identify the elements that undergo changes in oxidation number.
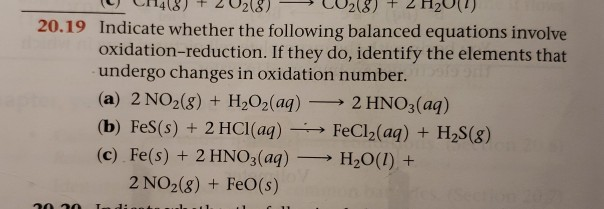
. If they do identify the elements that undergo changes in oxidation number. Complete and balance the following half-reactions. Chickenbird9861 is waiting for your help.
If they do identify the elements that undergo changes in oxidation number. PBr3l 3H20l H3P03aq 3HBr aq No reciox b. Were as to indicate whether the following balanced equations involve oxidation production.
If they do Identify the elements that undergo changes in oxidation number. Indicate whether the following balanced equations involve oxidation reduction. Indicate whether the following balanced equations involve oxidation-reduction.
If they do identify the elements. Indicate whether the following balanced equations involve oxidation-reduction. Since Cus Cu 2 aq 2 e E oxidation 0340 V then E Rh 3 Rh 0340 V.
If they do ended find the elements that undergo changes in oxidation. Indicate whether the following balanced equations involve oxidation-reduction. If they do identify the elements that undergo changes in oxidation number.
E cell E oxidation E reduction E Rh 3 Rh E Cu 2 Cu 0. Since Ags Ag aq e E oxidation 0800 V then E Rh 3 Rh 0800 V. If they do identify the elements that undergo changes in oxidation number.
A 2AgNO3 aq CoCl2aq ------. In each indicate whether the half-reaction is an oxidation or a reduction. Indicate whether the following balanced equations involve oxidation-reduction.
2AgCl s CoNO32 aq b 2PbO2 s ------. - 2AgNO3 aqCoCl2 aq2AgCl sCo NO32 aq -. If they do identify the elements that undergo changes in oxidation number.
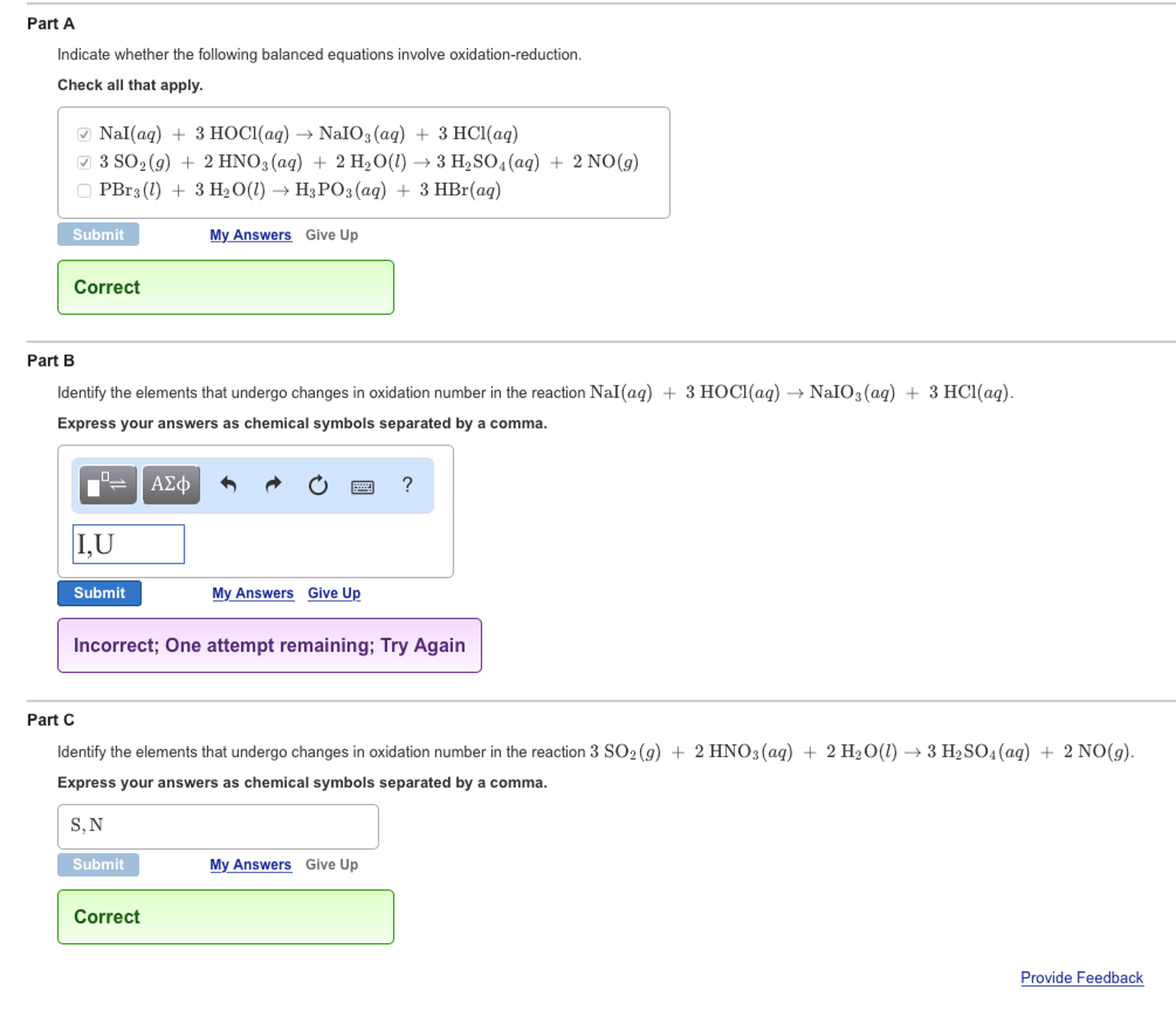
3S02 g 2HN03 aq 2H20 l 3H2S04aq 2NO g Il S YIU reduced. Indicate whether the following balanced equations involve oxidation-reduction. Indicate whether the following balanced equations involve oxidation-reduction.
If they do identify the elements that undergo changes in oxidation number. Find step-by-step Chemistry solutions and your answer to the following textbook question. Operatorname Sn 2 a q longrightarrow operatorname Sn 4 a q acidic solution.
Indicate whether the following balanced equations involve oxidation-reduction. Add your answer and earn points. Indicate whether the following balanced equations involve oxidation-reduction.
Complete and balance the following half-reactions. In each case indicate whether the half-reaction is an oxidation or a reduction. APBr3l 3H2OlH3PO3aq 3HBraq bNaIaq 3HOClaqNaIO3aq HClaq c3SO2g 2HNO3aq 2H2Ol3H2SO4aq 2NOg.
Indicate whether the following balanced equations involve oxidation-reduction. So for part A just glancing at the change of oxygen from a peroxide um to Apollo atomic ion its not a peroxide. 2PbO O2 g c 2H2SO4 aq 2NaBr s -------.
A Mo3 aq Mo s acidic b H2SO3 aq SO42- aq acidic c NO3- aq NO g acidic d O2 g H2O l acidic e O2 g H2O l basic f Mn2 aq MnO2 s basic. PBr_3 l 3H_2O lRight Arrow H_3PO_3 aq 3 HBr aq Nal aq 3 HOCl aq Right Arrow NalO_3 aq 3 HCl aq 3SO_2 g 2HNO_3 aq 2H_2O lRight Arrow 3 H_2SO_4 aq 2 NO g 2H_2SO_4 aq. Br2 l SO2g Na2SO4aq 2H2O l.
Indicate whether the following balanced equations involve oxidation-reduction. A PBr3 l 3 H2O l H3PO3 aq 3 HBr aq b 3 SO2 g 2 HNO3 aq 2 H2O l 3 H2SO4 aq 2 NO g. - 2AgNO3 aqCoCl2 aq2AgCl sCo NO32 aq - 2H2SO4 aq2NaBr sBr2 lSO2 gNa2SO4 aq2H2O l - 2PbO2 s2PbO sO2 g Question.
Nal aq 3HOCl aq Na O aq 3HCl aq CI reduced c. Indicate whether the following balanced equations involve oxidation-reduction. If they do identify the elements that undergo changes in oxidation number.
So first uh in this the first reaction which were given here is to silver nitrate. Classify each matter correctly as element compound homogeneous mixture and heterogeneous mixture. E cell E oxidation E reduction E Rh 3 Rh E Ag Ag 0.
NaI aq 3HOCl aq NaIO aq 3HCl aq. PBr3l 3H2Ol H3PO3aq 3HBr aq b. 4 Rh 3 aq Ags NR E cell 0.
Up to 256 cash back Indicate whether the following balanced equations involve oxidation-reduction. Indicate whether the following balanced equations involve oxidation-reduction. Check all that apply.
TF A reductant or reducing agent is reduced in a chemical reaction. Check all that apply. Solution for Indicate whether the balanced equation involves oxidationreduction.
If they do identify the elements that undergo changes in oxidation number. A P B r 3 l 3 H 2 O l H 3 P O 3 a q 3 H B r a q b N a I a q 3 H N O l a q N a I O 3 a q 3 H C l a q c 3 S O 2 g 2 H N O 3 a q 2 H 2 O l 3 H 2 S O 4 a q 2 N O g. 2AgNO 3 aq CoCl 2 aq 2AgCls CoNO 3 2 aq 2PbO 2 s 2 PbOs O 2 g 2H 2 SO 4 aq 2NaBrs Br 2 l SO 2 g Na 2 SO 4 aq 2 H 2 Ol One Class.
Indicate whether the following balanced equatins involve oxidation-reduction. For each of the following balanced oxidation-reduction reactions i identify the oxidation numbers for all the elements in the reactants and products and ii state the total number of electrons transferred in each reaction. APBr3 l 3H2O lH3PO3 aq 3HBr aq bNaI aq 3HOCl aqNaIO3 aq HCl aq c3SO2 g 2HNO3 aq 2H2O l3H2SO4 aq 2NO g d 2H2SO4 aq 2NaBr sBr2 l Question.
Indicate whether the following balanced equations involve oxidation-reduction. Indicate whether the following balanced equations involve oxidation-reduction. A PBr3l 3 H2Ol H3PO3aq 3 HBraq b NaIaq 3 HOClaq NaIO3aq 3 HClaq.
A Ba2aq 2OH-aq H2O2aq 2ClO2aq yields BaClO22 s 2H2O l O2 g b 2H2SO4aq 2NaBrs yields Br2l SO2 g Na2SO4 aq 2 H2O l. A - 2AgNO3 aqCoCl2 aq2AgCl sCo NO32 aq b - 2H2SO4 aq2NaBr.
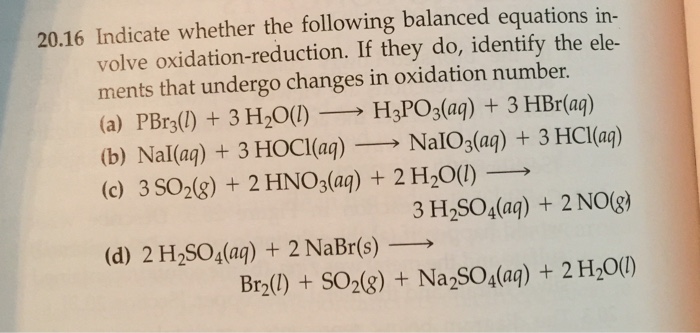
Solved Indicate Whether The Following Balanced Equations Chegg Com
Solved Indicate Whether The Following Balanced Equations Chegg Com

Solved Problem 20 18 Part A Indicate Whether The Following Chegg Com
0 Comments